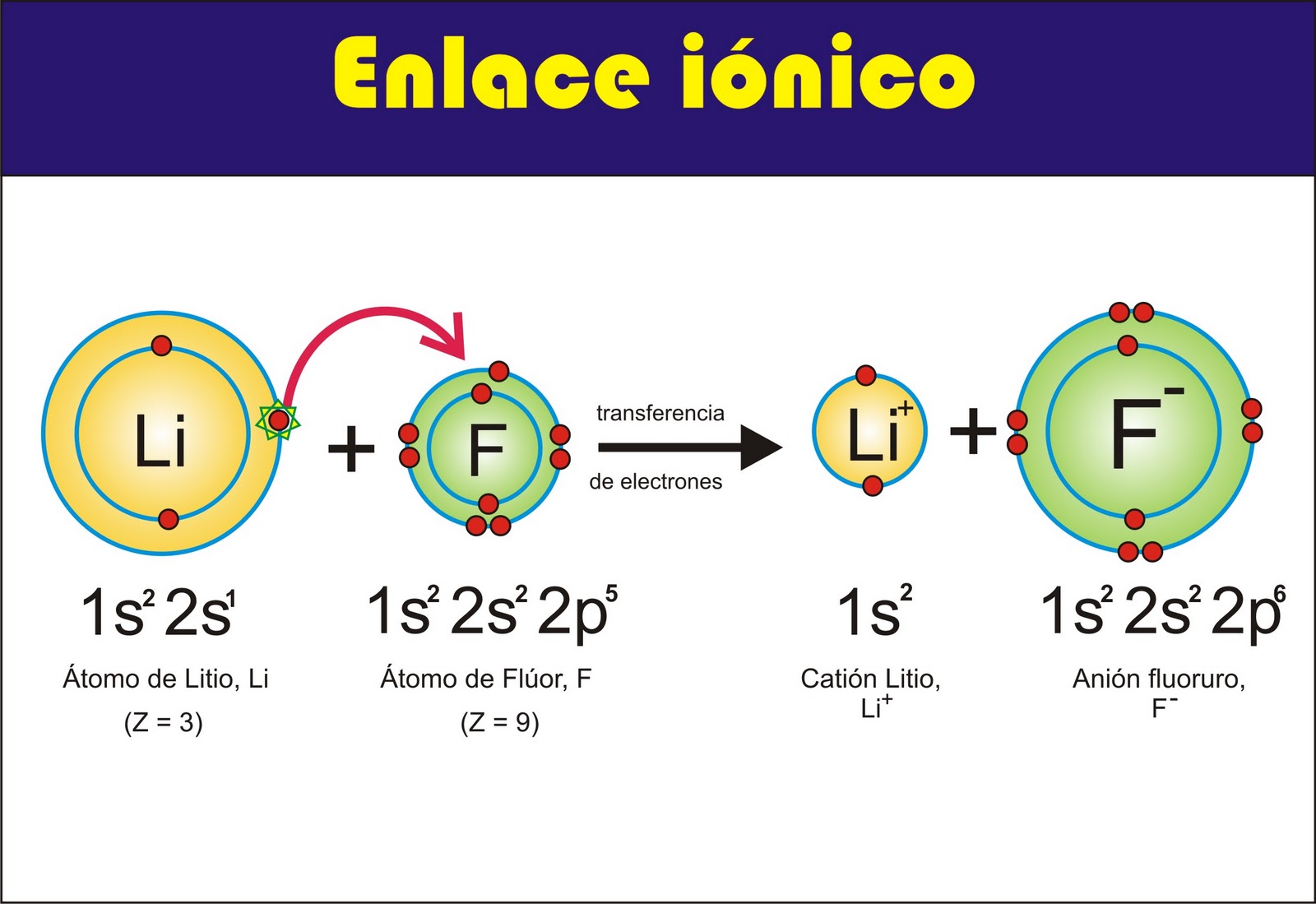
Ever wondered what makes ionic and covalent bonds stick together? Discover the fascinating world of chemical bonding through a journey of "ionic and covalent bond exercises"!
In chemistry, bonds form the foundation of molecular structures. Among these bonds, ionic and covalent bonds stand out as the two main types. Ionic bonds result from the electrostatic attraction between oppositely charged ions, while covalent bonds arise from the sharing of electron pairs between atoms.
Delving into "ionic and covalent bond exercises" unveils the principles that govern these bonds. Through practice problems and interactive simulations, students can visualize the formation and properties of ionic and covalent compounds. By manipulating charges and electronegativity values, they gain a deeper understanding of bond polarity and the resulting molecular shapes.
The significance of these exercises extends beyond theoretical knowledge. They equip students with the skills to predict and analyze chemical reactions, design new materials, and explore the vast field of molecular science. Moreover, these exercises foster critical thinking, problem-solving abilities, and a profound appreciation for the intricate world of chemical bonding.
Ionic and Covalent Bond Exercises
Ionic and covalent bond exercises provide a gateway to understanding the fundamental principles of chemical bonding. These exercises encompass a range of key aspects, each offering a unique perspective on this crucial topic:
- Interactive Learning: Hands-on simulations and problem-solving challenges engage students in the dynamic process of bond formation.
- Visualizing Bond Properties: Exercises illustrate the geometry, polarity, and strength of different types of bonds, fostering a deeper comprehension of their characteristics.
- Predicting Reactivity: By manipulating charges and electronegativity, students develop the ability to forecast the reactivity of compounds and design new materials.
- Molecular Architecture: Exercises explore how bond angles and lengths influence molecular shapes and properties, providing insights into the structure-function relationships of molecules.
- Real-World Applications: Connections to real-world phenomena, such as the behavior of ionic liquids and the formation of semiconductors, highlight the practical significance of bond exercises.
- Critical Thinking and Problem-Solving: These exercises challenge students to think critically, analyze data, and solve problems related to chemical bonding, honing their scientific inquiry skills.
In essence, ionic and covalent bond exercises are not merely academic pursuits but invaluable tools for unlocking the mysteries of chemical bonding. They empower students to explore the intricate dance of electrons and ions, and to apply their understanding to a wide range of scientific endeavors.
Interactive Learning
Interactive learning, a cornerstone of "ejemplos de ejercicios de enlaces ionicos y covalentes" (examples of ionic and covalent bond exercises), plays a pivotal role in fostering a deep understanding of chemical bonding. Hands-on simulations and problem-solving challenges immerse students in the dynamic process of bond formation, enabling them to visualize and manipulate molecular structures.
These interactive exercises provide a tangible and engaging platform for students to explore the interplay of charges and electronegativity, the factors that govern bond polarity and molecular geometry. By actively participating in simulations and solving problems, students develop a profound comprehension of the forces that shape molecular architecture and dictate chemical reactivity.
The practical significance of this understanding extends far beyond the classroom. In the field of materials science, for instance, a thorough grasp of bond formation is essential for designing new materials with tailored properties. From lightweight alloys to high-efficiency semiconductors, the ability to manipulate and predict bond behavior holds the key to technological advancements.
Interactive learning, therefore, serves as a fundamental component of "ejemplos de ejercicios de enlaces ionicos y covalentes," equipping students with the skills and knowledge necessary to unravel the intricacies of chemical bonding and harness its power for scientific discovery and technological innovation.
Visualizing Bond Properties
In the realm of "ejemplos de ejercicios de enlaces ionicos y covalentes" (examples of ionic and covalent bond exercises), visualizing bond properties stands as a pivotal component, illuminating the intricate characteristics of chemical bonds. These exercises employ a range of techniques, including interactive simulations and problem-solving challenges, to provide students with a tangible and immersive learning experience.
By visualizing the geometry, polarity, and strength of different types of bonds, students develop a profound understanding of how these properties influence molecular structure and reactivity. For instance, the visualization of bond polarity enables students to comprehend the uneven distribution of charge within a molecule, which is crucial for predicting molecular behavior and interactions. Similarly, visualizing bond strength allows students to grasp the relative stability of different bonds, a factor that governs chemical reactivity and the design of new materials.
The practical significance of visualizing bond properties extends to various scientific disciplines. In biochemistry, for example, understanding bond polarity is essential for deciphering the structure and function of biological molecules, such as proteins and nucleic acids. In materials science, visualizing bond strength is crucial for designing materials with tailored properties, such as high strength, low thermal conductivity, or specific electrical properties.
In essence, visualizing bond properties through "ejemplos de ejercicios de enlaces ionicos y covalentes" empowers students with the ability to visualize and comprehend the fundamental building blocks of matter. This understanding serves as a cornerstone for scientific discovery and technological innovation, driving progress in fields as diverse as medicine, materials science, and energy research.
Predicting Reactivity
Within the realm of "ejemplos de ejercicios de enlaces ionicos y covalentes" (examples of ionic and covalent bond exercises), predicting reactivity occupies a central position, empowering students to unravel the chemical behavior of compounds and design materials with tailored properties.
By manipulating charges and electronegativity, students gain the ability to forecast the reactivity of compounds. Electronegativity, a measure of an atom's ability to attract electrons, plays a crucial role in determining bond polarity and, consequently, reactivity. Understanding electronegativity allows students to predict the distribution of charge within a molecule and infer its reactivity towards other molecules.
This predictive capability extends to the design of new materials. By manipulating charges and electronegativity, scientists can engineer materials with specific properties, such as high strength, low thermal conductivity, or tailored electrical properties. This understanding is particularly valuable in fields such as materials science and nanotechnology, where the ability to design materials with desired properties is essential for technological advancements.
In summary, predicting reactivity through the manipulation of charges and electronegativity is a key component of "ejemplos de ejercicios de enlaces ionicos y covalentes." It empowers students to understand and predict the behavior of compounds, enabling them to contribute to the design of novel materials and drive scientific progress.
Molecular Architecture
Delving into the realm of "Molecular Architecture," these exercises provide a deeper understanding of the intricate interplay between bond angles, lengths, molecular shapes, and properties. This knowledge is crucial for comprehending the structure-function relationships of molecules, which underpin their behavior and applications in various scientific fields.
- Shape Determination: By analyzing bond angles and lengths, students can determine the molecular shape, which influences properties such as polarity, solubility, and reactivity. For instance, the tetrahedral shape of methane results from the four equivalent C-H bonds, while the linear shape of carbon dioxide arises from the two C=O double bonds.
- Property Prediction: Understanding molecular shapes enables the prediction of various properties. For example, the bent shape of water allows for hydrogen bonding, leading to its high boiling point and unique solvent properties.
- Biomolecular Interactions: In biological systems, molecular shapes play a vital role in molecular recognition and interactions. For example, the specific shape of enzymes allows them to bind to specific substrates, enabling efficient catalysis.
- Drug Design: In the field of drug design, understanding molecular shapes is crucial for developing drugs that can interact with target molecules. By manipulating bond angles and lengths, scientists can design drugs that are more effective and have fewer side effects.
In conclusion, the exploration of molecular architecture through bond angles and lengths provides invaluable insights into the structure-function relationships of molecules. This knowledge empowers students and researchers to understand and manipulate molecular properties, paving the way for advancements in fields such as materials science, drug design, and biochemistry.
Real-World Applications
The realm of "Real-World Applications" forges an unbreakable bond between "ejemplos de ejercicios de enlaces ionicos y covalentes" (examples of ionic and covalent bond exercises) and the tangible world around us. These exercises transcend theoretical boundaries, extending their impact to a myriad of practical applications and phenomena.
- Ionic Liquids: Ionic liquids, a class of molten salts, exhibit remarkable properties such as negligible vapor pressure and high ionic conductivity. Understanding the nature of ionic bonding through exercises enables the design of ionic liquids with tailored properties for applications in diverse fields, including electrochemistry, catalysis, and green solvents.
- Semiconductors: Semiconductors, the foundation of modern electronics, owe their unique properties to the precise arrangement and bonding of atoms. Exercises on covalent bonding provide insights into the formation and properties of semiconductors, enabling the development of advanced electronic devices, solar cells, and other cutting-edge technologies.
- Biological Systems: The behavior of ionic and covalent bonds is central to understanding the structure and function of biological molecules. Exercises in this area provide a deeper comprehension of enzyme catalysis, protein folding, and the interactions between biomolecules, paving the way for advancements in biotechnology and medicine.
- Materials Science: The design of new materials with tailored properties relies heavily on an understanding of chemical bonding. Exercises on bond angles, lengths, and strengths empower scientists to engineer materials with enhanced mechanical properties, thermal conductivity, and electrical properties for applications in aerospace, energy storage, and construction.
In conclusion, "Real-World Applications" solidifies the practical significance of "ejemplos de ejercicios de enlaces ionicos y covalentes." These exercises serve as a bridge between theoretical concepts and real-world phenomena, equipping students and researchers with the knowledge and skills to address complex challenges and drive scientific progress.
Critical Thinking and Problem-Solving
Within the realm of "ejemplos de ejercicios de enlaces ionicos y covalentes" (examples of ionic and covalent bond exercises), critical thinking and problem-solving stand as indispensable cornerstones, fostering a deep understanding of chemical bonding and its applications.
- Analytical Reasoning: These exercises require students to analyze data, identify patterns, and draw logical conclusions about chemical bonding. This enhances their ability to interpret experimental results and make informed predictions.
- Deductive Logic: Students utilize deductive reasoning to apply general principles of chemical bonding to specific scenarios. This strengthens their ability to solve problems and make valid inferences about molecular structures and properties.
- Hypothesis Testing: Exercises often involve formulating hypotheses and designing experiments to test them. This develops students' experimental design skills and their ability to evaluate the validity of scientific claims.
- Communication: Effectively communicating scientific findings is crucial. These exercises encourage students to present their solutions and defend their reasoning, honing their communication and presentation skills.
In summary, the integration of critical thinking and problem-solving into "ejemplos de ejercicios de enlaces ionicos y covalentes" empowers students to develop a comprehensive understanding of chemical bonding. These exercises foster analytical reasoning, deductive logic, hypothesis testing, and communication skills, equipping students to excel in scientific inquiry and problem-solving.
FAQs on "Ionic and Covalent Bond Exercises"
This section addresses common queries and misconceptions regarding "ejemplos de ejercicios de enlaces ionicos y covalentes" (examples of ionic and covalent bond exercises) to provide a comprehensive understanding of their significance and applications.
Question 1: What is the purpose of ionic and covalent bond exercises?
Answer: These exercises facilitate a deeper understanding of the fundamental principles of chemical bonding, enabling students to visualize and analyze the formation and properties of ionic and covalent compounds.
Question 2: How do these exercises enhance critical thinking skills?
Answer: By challenging students to analyze data, formulate hypotheses, and solve problems related to chemical bonding, these exercises foster analytical reasoning, deductive logic, and problem-solving abilities.
Question 3: Are these exercises solely theoretical, or do they have practical applications?
Answer: While these exercises provide a solid theoretical foundation, they also highlight practical applications in diverse fields such as materials science, drug design, and biological systems, demonstrating the relevance of chemical bonding in the real world.
Question 4: How do these exercises contribute to scientific research?
Answer: By equipping students with a comprehensive understanding of chemical bonding, these exercises empower them to contribute to scientific research by designing new materials, developing novel drugs, and advancing our knowledge of molecular interactions.
Question 5: Are these exercises suitable for students of all levels?
Answer: While the complexity of exercises may vary, the fundamental concepts and principles explored are accessible to students of various levels, providing a pathway for progressive learning and understanding.
Question 6: How can I incorporate these exercises into my teaching or learning experience?
Answer: These exercises can be seamlessly integrated into chemistry curricula, utilized as interactive learning modules, or employed as self-study resources to reinforce classroom learning and promote a deeper understanding of chemical bonding.
Summary: "Ionic and covalent bond exercises" play a crucial role in developing a comprehensive understanding of chemical bonding, fostering critical thinking skills, and providing a foundation for scientific research and practical applications. By engaging in these exercises, students gain valuable insights into the fundamental principles that govern the formation and properties of chemical compounds.
Transition to the next article section: Having explored the significance and applications of "ionic and covalent bond exercises," we now delve into specific examples and problem-solving strategies to further enhance our understanding of these essential concepts in chemistry.
Conclusin
A lo largo de este artculo, hemos explorado la importancia y aplicaciones de los "ejemplos de ejercicios de enlaces ionicos y covalentes". Estos ejercicios proporcionan una base slida para comprender los principios fundamentales de la unin qumica, fomentan el pensamiento crtico y sientan las bases para la investigacin cientfica y las aplicaciones prcticas.
Al participar en estos ejercicios, los estudiantes obtienen informacin valiosa sobre los principios fundamentales que gobiernan la formacin y las propiedades de los compuestos qumicos. El estudio de los enlaces inicos y covalentes no slo ampla nuestro conocimiento terico, sino que tambin tiene implicaciones significativas en campos como el diseo de materiales, el desarrollo de frmacos y la comprensin de los sistemas biolgicos.
A medida que continuamos explorando el fascinante mundo de la unin qumica, estos ejercicios seguirn desempeando un papel crucial en el avance de nuestro conocimiento y en la configuracin de nuestro futuro cientfico y tecnolgico.
What Is The Noun Communication - Definition And Examples
Uncover The Real-Life Department Store Behind "Miracle On 34th Street"
Central Nervous System: Structure And Functions In A Nutshell